61.WHAT IS THE CARBON CYCLE?
Of all the chemical elements known to man, perhaps the most interesting one is carbon. Did you know, for example, that in the form of crystal it gives us one of our most valuable gems, the diamond? As graphite, carbon forms the lead of lead pencils. And coal, the source of much of the heat and power of this machine age, is mostly carbon.
But even more important is the fact that carbon is so vital to life. The bodies of all living things are made up of compounds containing carbon. In fact, scientists believe that where carbon is found in any quantity in the earth, there life has probably existed.
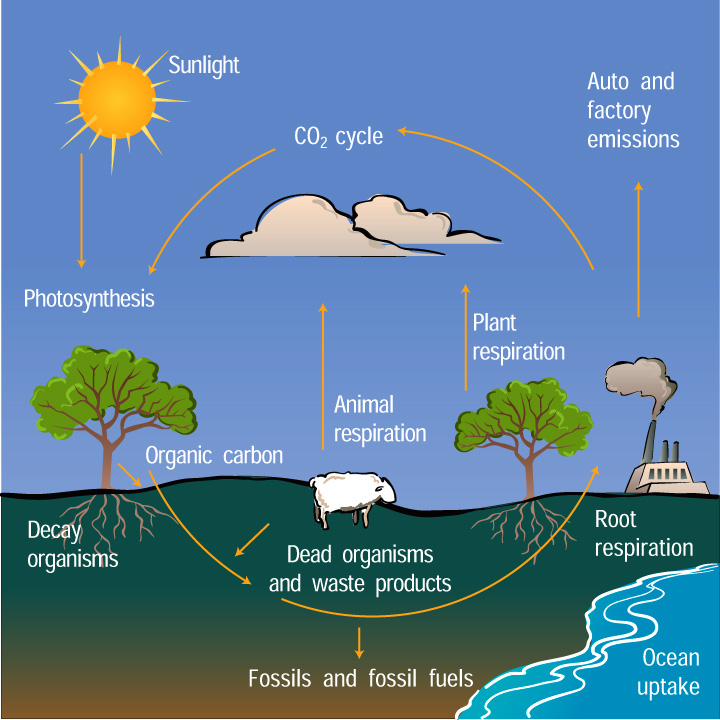
The carbon cycle is the process in which carbon is continuously being removed, used, and replaced by living things. Here is how it works. There is carbon dioxide in the air. Plants secure carbon from this gas and use it in building up their roots, stems, and leaves. Animals get carbon for food from the plants, in the form of vegetables, fruits, or cereals. At the same time, carbon dioxide is being returned to the air, especially by animals’ breathing, and by the burning or decay of plants. The carbon cycle is thus completed.
When elements combine, we have a compound. The number of carbon compounds that we know so far is enormous—more than 200,000 of them! All the other elements together do not form nearly as many compounds as the single element carbon. The reason for this is that the carbon atom can join with the atoms of other elements in so many different ways and can form rings and chains in combination with other carbon atoms.
You come in contact with or use carbon compounds constantly in your daily life. You breathe in a little carbon dioxide and breathe out much more. And most fuels, foods, drugs, plastics, perfumes (and dozens upon dozens of other products) are carbon compounds!


Leave a Reply