42.WHERE DOES WATER GO WHEN IT DRIES UP?
You look out on the street or road and you see water. An hour later in bright sunshine, it is gone! Or wet clothes are hung out on a line, and by the end of the day, they are dry. Where did the water go?
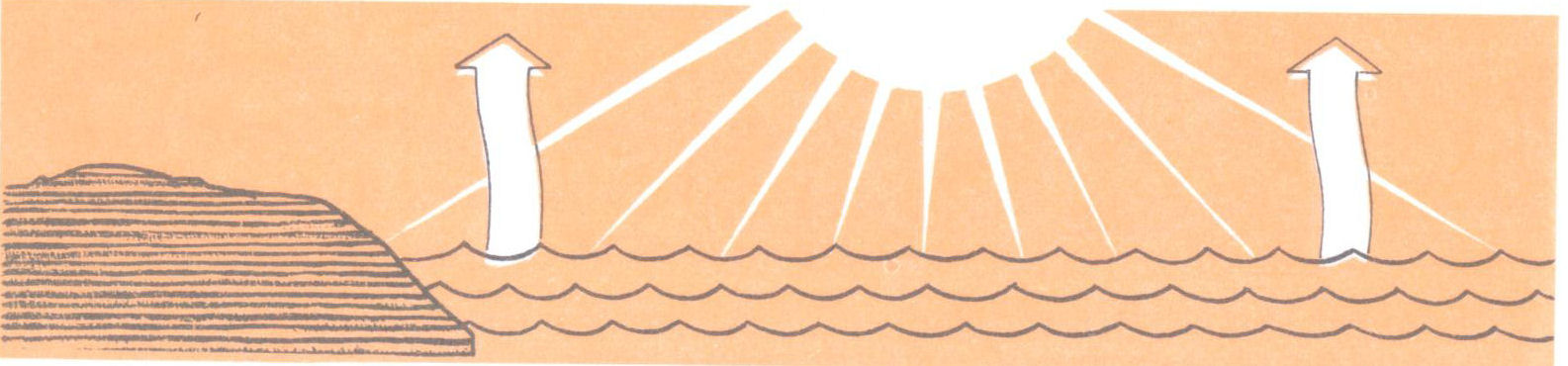
We say the water evaporates. But what does this mean? Evaporation is the process by which a liquid that is exposed to air gradually becomes a gas or vapor. Many liquids evaporate quite quickly, much more quickly than water. This is true of alcohol, gasoline, and ammonia. Some liquids, such as mercury, evaporate very slowly.
What causes evaporation? To understand this, we must know something about the nature of matter. As we know, every substance is made up of molecules. Two forces are at work on these molecules. One is cohesion, which draws them together. The other is the heat motion of the individual molecules, which makes them fly apart.
When the force of cohesion is stronger, the substance is a solid. When the heat motion is so strong that it overcomes cohesion, the substance is a gas. When the two forces are balanced fairly evenly, we have a liquid.
Water, of course, is a liquid. But at the surface of the liquid, there are molecules that are moving so rapidly that they fly into space and escape the force of cohesion. This process of escaping molecules is evaporation.
Why does water evaporate more quickly in sunshine or when heat is applied? The greater the heat, the more heat motion there is in the liquid. This means more molecules will have enough speed to escape. When the fastest molecules escape, the average speed of those left behind is lowered. So the remaining liquid is cooled by evaporation.
So when water dries up, what has really happened is that it has become a gas or vapor and part of the air.


Leave a Reply